UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of principal executive offices) | (Zip Code) |
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure. |
Third Harmonic Bio, Inc. (the “Company”) is furnishing its corporate presentation, which it intends to use in conferences and meetings. The full copy of the Company’s corporate presentation is filed as Exhibit 99.1 hereto. The corporate presentation will also be available on the Company’s website in the Investors & Media section at https://ir.thirdharmonicbio.com.
The information furnished in Exhibit 99.1 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1934, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Corporate Presentation. | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| THIRD HARMONIC BIO, INC. | ||||||
| Date: July 25, 2023 | By: | /s/ Robert Ho | ||||
| Robert Ho | ||||||
| Chief Financial Officer | ||||||

FOCUSED On advancing the next wave of medicine for inflammatory diseases JULY 2023 ©2023 THIRD HARMONIC BIO Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995. Any statements made in this presentation that are not statements of historical fact, including statements about our beliefs and expectations, are forward-looking statements and should be evaluated as such. Forward-looking statements include information concerning the anticipated profile and efficacy of our new product candidate, the expected development and timeline for clinical and non-clinical studies of THB335 candidate, the timing of presentations on Phase 1a HV data, and the filing of an IND application for THB335 candidate, the market potential and addressable patient population for an oral KIT inhibitor, our intellectual property strategy for KIT inhibitors, and our possible or assumed future results of operations, including descriptions of our business plan and strategies. These statements often include words such as “anticipate,” “expect,” “suggests,” “plan,” “believe,” “intend,” “estimates,” “targets,” “projects,” “should,” “could,” “would,” “may,” “will,” “forecast” and other similar expressions. These forward-looking statements are contained throughout this presentation. We base these forward-looking statements on our current expectations, plans and assumptions that we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate under the circumstances at such time. As you read and consider this presentation, you should understand that these statements are not guarantees of future performance or results. The forward-looking statements are subject to and involve risks, uncertainties and assumptions, and you should not place undue reliance on these forward-looking statements. Although we believe that these forward-looking statements are based on reasonable assumptions at the time they are made, you should be aware that many factors could affect our actual results or results of operations and could cause actual results to differ materially from those expressed in the forward-looking statements. Factors that may materially affect such forward-looking statements include: our limited operating history and that we have not completed any clinical trials beyond Phase 1 and have not had any product candidates approved for commercial sale; our significant net losses incurred since inception and the likelihood of incurring additional losses for the foreseeable future; our need for substantial additional funding; the early stage of development of our programs and the possibility they may fail in development; our future performance is substantially dependent on our ability to identify and develop future product candidates; legal and regulatory risks; and intellectual property-related risks, among others. Additional risks and uncertainties that could affect our financial results and business are more fully described under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the three months ended March 31, 2023, filed with the SEC on May 11, 2023, and our other SEC filings, which are available on the Investor & Media page of our website at https://ir.thirdharmonicbio.com/ and on the SEC’s website at www.sec.gov. These cautionary statements should not be construed by you to be exhaustive and are made only as of the date of this presentation. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

“PIPELINE-IN-A-TARGET” POTENTIAL SELECTIVE ORAL KIT INHIBITORS KIT: A NOVEL, CLINICALLY VALIDATED TARGET LARGE ESTABLISHED MARKETS WITH HIGH UNMET NEED Third Harmonic Bio: Focused on KIT Inhibition to Treat Mast Cell-Mediated Inflammatory Diseases Millions of patients living with severe mast cell-mediated diseases; high residual need despite multiple approved products Clinical validation of KIT as potentially transformative target for mast cell-mediated diseases Highly selective oral small molecule with opportunity to optimize therapeutic index and offer patient convenience over injectables Potential to be an attractive treatment option for a range of dermal, airway and GI inflammatory diseases Respiratory CRSwNP Skin / Eye Mast Cell-Mediated Diseases Gastrointestinal Food allergy EoE IBD IBS Allergic conjunctivitis Chronic urticaria Atopic dermatitis Asthma

MAST CELL Receptor-binding agonists IgE Complement Neuropeptides Microbial products Cytokines TSLP Chemokines Physical activators Temperature Pressure Cell-cell contact Pre-formed mediators Histamine IL-4, IL-13 TNF, GM-CSF Proteases Serotonin Heparin Newly synthesized mediators Prostaglandins Leukotrienes Cytokines Chemokines Neuropeptides PAF, free radicals Lymphocyte ligands MANY MEDIATORS MANY ACTIVATORS OPTIMAL INTERVENTION POINT The Mast Cell Itself Anti-histamines Dupilumab Anti-leukotrienes Mast Cells are a Fulcrum of Inflammation Current therapeutic approaches are mechanistically limited Omalizumab Tezepelumab IgE Receptor Antigen Histamines Degranulation

KIT is the Master Regulator of Mast Cell Function and Survival Intracellular small molecule approach to KIT inhibition offers multiple potential therapeutic advantages KIT Master regulator of mast cell proliferation, migration, activation and survival KIT inhibition drives both mast cell inactivation and depletion Dimerization SCF dimer KIT receptor Growth, Differentiation, Survival, Chemotaxis, Cytokine Production Function, Degranulation PLC�� JAK2/STAT PI3K/Akt Ras/Raf/Mek/Erk P P P P P P P P Small Molecule Intracellular Small Molecule Inhibition Potential for therapeutic index optimization Patient and medical practice convenience Avoids risk of MAb-mediated mast cell activation/anaphylaxis

THB001 Clinical results from first generation oral KIT inhibitor

First-Generation Product Candidate: THB001 Early results support the potential of oral KIT inhibition and inform next-gen development THB001 demonstrated high potency and selectivity for KIT à mast cell depletion and disease model efficacy in multiple nonclinical studies Phase 1a 14-day healthy volunteer study completed Dose-dependent increases in THB001 plasma exposure and decreases in serum tryptase Mild decreases in hematologic parameters and hair color change consistent with on-target effects of KIT inhibition 14-day study results largely predictive of serum tryptase and hematologic effects seen in 12-week study THB001 Phase 1 study results: Rapid and dose-dependent drops in serum tryptase PBO 200mg QD 200mg BID 400mg BID 500mg QD FED Serum Tryptase (% Baseline) End of treatment PBO = placebo; Mean percent change from baseline calculated using “0” for values <LLOQ (1.0 )

THB001 Discontinued Phase 1b Chronic Inducible Urticaria1 Study Overview Dose escalation study designed to interrogate potential for therapeutic index optimization Design and Objectives 3 doses (1:1:1) of THB001 (total N=30) for 12 weeks Pharmacokinetics and serum tryptase levels Mean reduction in critical temperature threshold (CTT) Study Disposition Enrolled 5 subjects in 200mg BID dose cohort before study discontinuation 1 subject completed 12 weeks of treatment 2 subjects discontinued at week 8 due to drug-induced liver injury (DILI) AEs 2 remaining subjects were discontinued from study drug at weeks 3 and 4 and were followed for safety SCREENING 4 Weeks THB001 200mg BID (n=10) THB001 300mg BID (n=10) THB001 400mg BID (n=10) SRC Study Schematic SRC 12 WEEKS TX Follow-up 4 WEEKS Follow-up 4 WEEKS Follow-up 4 WEEKS 12 WEEKS TX 12 WEEKS TX SRC=safety review committee 1. CINDU
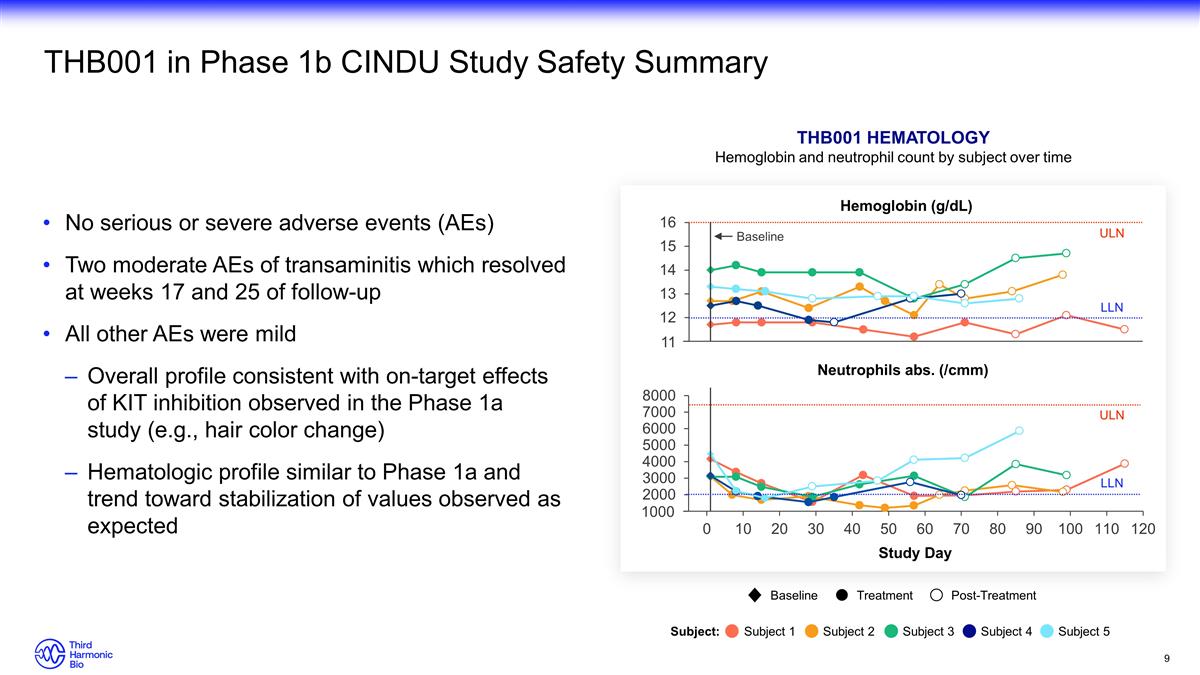
11 1000 THB001 in Phase 1b CINDU Study Safety Summary No serious or severe adverse events (AEs) Two moderate AEs of transaminitis which resolved at weeks 17 and 25 of follow-up All other AEs were mild Overall profile consistent with on-target effects of KIT inhibition observed in the Phase 1a study (e.g., hair color change) Hematologic profile similar to Phase 1a and trend toward stabilization of values observed as expected ULN ULN LLN LLN Baseline THB001 Hematology Hemoglobin and neutrophil count by subject over time Study Day Baseline Treatment Post-Treatment Subject: Subject 1 Subject 2 Subject 3 Subject 4 Subject 5

THB001 Generated Responses at Lowest Planned Dose in Phase 1b Study 4 of 5 subjects reached partial (n=2) or complete (n=2) Critical Temperature Threshold responses Rapid tryptase reduction: -83% mean change from baseline by week 1 largely consistent with Phase 1a results Strong correlation between serum tryptase reduction and clinical response consistent with other published urticaria clinical data 4/5 patients achieved clinical response despite early termination of study Note: Negative TempTest results (complete response) are shown at 3° C. Serum Tryptase values below lower limit of quantification are shown at 0 µg/L. Empty circles indicate results post treatment. TempTest °C Serum Tryptase µg/L Serum lower limit of quantitation = 1 µg/L TempTest complete response ≤ 4°C SUBJECT 5 >> COMPLETE RESPONSE SUBJECT 4 >> COMPLETE RESPONSE SUBJECT 2>> PARTIAL RESPONSE SUBJECT 1 >> PARTIAL RESPONSE SUBJECT 3>> NO RESPONSE Study Day Baseline Treatment Post-Treatment

Understanding Hepatic Effects of THB001 Generating mechanistic understanding allows for differentiation of next-generation candidate Conducting studies characterizing liver metabolism and phenotypic effects of THB001 Employing a comprehensive approach: Assessing evidence for off-target biology liabilities Charactering liver metabolism and potential for formation of reactive metabolites Identifying phenotypic effects associated with THB001 in advanced hepatic testing systems Applied learnings to next-generation compound screening and candidate selection

THB001 Shows Evidence for Formation of a Toxic Reactive Metabolite Three findings from mechanistic studies provide potential basis for observed transaminitis Studies identify major metabolite in human plasma which is formed via a reactive intermediate Metabolite present at higher levels in human plasma than in toxicology animal species Detected glutathione (GSH) conjugate metabolites in human urine samples from Phase 1b study Indicates potential to cause oxidative stress Measured high levels1 of protein adduct formation in vitro with radiolabeled THB001 Indicates potential to irreversibly inhibit protein function and/or trigger immune response [14C] THB001 Covalent Protein Adduct Formation in Human Liver Microsomes Values mean of n=2 or 3 independent donor pools each done in duplicate except ABT that is from a single donor pool. ABT, 1-aminobenzotriazole. 1 Published literature cut-off: Evans D.C. et al. Chem Res Toxicol 2004 POSITIVE CONTROL RELATIVE METABOLITE SCAVENGER CYP INHIBITOR Literature cut-off p<0.05

THB335 Next generation oral KIT inhibitor

THB335 Potent and Selective Small Molecule KIT Inhibitor Maintained Kinase Inhibition Profile to THB001 but Lacking Evidence for Reactive Metabolite Formation THB001 THB335 KIT IC50 23 nM 9.5 nM PDGFRa Selectivity >100-fold >100-fold CSF1R Selectivity 65-fold >100-fold Off-target cell viability No effect at 3 µM Brain-to-plasma ratio 0.9 to 1.2 <0.1 Reactive intermediate metabolite Yes No Glutathione adduct formation Yes No Comparison of key kinase and metabolic pathway parameters THB335 KinomeScan KIT Percent Control 0% 0.1% 0.1-1% 1-5% 10-35% > 35% 5-10% 467 Assays Tested 8 Interactions Mapped KinomeScan completed at 100 nM THB335 KIT and CSF1R IC50 determined by NanoBRET. PDGFR⍺ IC50 determined by homogeneous time resolved fluorescence (HTRF). Viability was assessed in cell lines dependent on CSF1R and PDGFRβ, respectively.
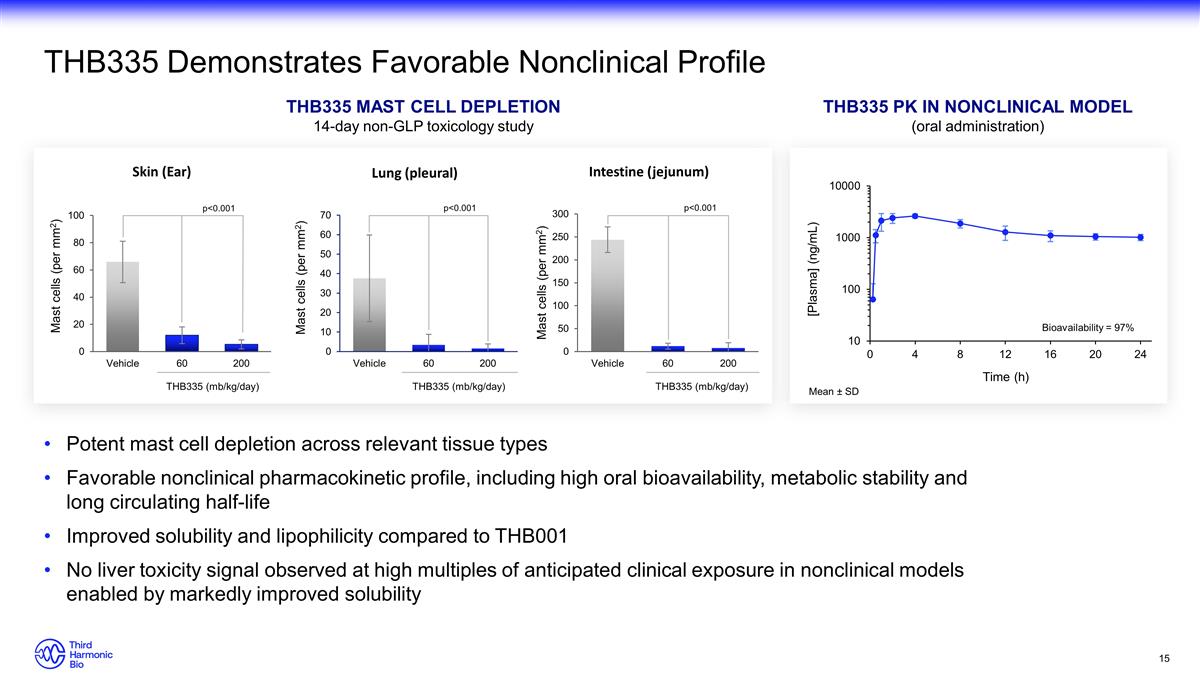
Potent mast cell depletion across relevant tissue types Favorable nonclinical pharmacokinetic profile, including high oral bioavailability, metabolic stability and long circulating half-life Improved solubility and lipophilicity compared to THB001 No liver toxicity signal observed at high multiples of anticipated clinical exposure in nonclinical models enabled by markedly improved solubility THB335 Demonstrates Favorable Nonclinical Profile THB335 Pk in NONCLINICAL MODEL (oral administration) Mean ± SD THB335 mast cell depletion 14-day non-GLP toxicology study Bioavailability = 97% THB335 (mb/kg/day) THB335 (mb/kg/day) THB335 (mb/kg/day) p<0.001 p<0.001

Third Harmonic Bio Next Steps Advancing THB355 back toward the clinic with a longer-term view toward franchise expansion THB335 U.S. IND filing and clinical trial initiation anticipated in 1H 2024 Targeting chronic spontaneous urticaria as initial clinical indication Planned expansion into additional mast-cell mediated inflammatory disorders at Phase 2 Medicinal chemistry, next-generation efforts continuing to support pipeline-in-a-target potential Maintaining focused operational strategy Selectively evaluating business development opportunities to expand portfolio Cash and cash equivalents of $282.2M as of March 31, 2023

ADVANCING the next wave of medicine for inflammatory diseases
